Instructions for Authors
CMJ is the official journal of the Trinidad & Tobago Medical Association and is published quarterly. It is national and regional in scope primarily; but in the process of expansion to publish international work. It is comprehensive in coverage and it publishes original, peer-reviewed articles on all branches of medicine, surgery, dentistry. We welcome submissions pertaining to both clinical sciences as well as basic sciences, provided their clinical relevance is well explained in the paper.
Author Guidelines
1. Before you start- Author responsibilities
Research and publication ethics
Caribbean Medical Journal strives to stringently adhere to all the core principles of research and publication ethics. This ensures not only strengthening of its trust with the readership but also promoting high quality scientific publications which have a sound ethical background
Any form of scientific misconduct is abhorred by the journal.
The following are the ethical requirements of the journal:
- All research papers submitted to CMJ must have a statement that it was approved by an Institutional Review Board / Ethics Committee. For clinical trials, any registry information and/or Approval ID number from the IRB must be included.
- Where informed consent is applicable, the paper must explicitly state that either informed consent was obtained from individual subjects or a ‘waiver’ of consent was approved by the IRB
- Case Reports must have a statement that informed consent was obtained from the patient or legal representative
- There should be a meticulous attempt to exclude any protected health and confidential information; photographs must be edited to avoid displaying the identity of the patient
- The corresponding author must assume the responsibility for the data as well the paper submitted, and any form of academic misconduct such as falsification, fabrication, using other’s data without permission, plagiarism of any kind will not be tolerated.
- Plagiarism of any type will not be permitted – by definition plagiarism is claiming someone else’s work as one’s own.
- Duplication of submission to different journals, whether in total or in part, including ‘salami’ publications (previously published papers modified with a little new information to convert it into a new paper) are also not permitted.
- Publications in the form of Abstracts, Conference Proceedings are acceptable (from the same study), but this must be notified during submission.
- If the journal editorial team (or the peer-reviewer, or if the journal receives a complaint at a later date) finds any academic misconduct, the journal will investigate the matter, if found true the paper will be retracted and the authors will be blacklisted for future submissions
- Authorship criteria (see below) must be strictly adhered to for naming a person as an author and any discrepancy at a later date will not be entertained
- The covering letter must explicitly state the contribution of each author, and that there is no conflict of interest between the authors and all authors have approved the final version
- The covering letter must also state that the paper is an independent work of the authors and permission was obtained for material borrowed from other sources (if any) and also the source has been explicitly quoted in the paper.
2. Preparing your manuscript – Instruction to Authors
Authorship criteria
All authors submitting their work must meet the requirements of authorship as set out in the guidelines of the International Committee of Medical Journal Editors (http://www.icmje.org/):
- Every author has made a substantial contribution to the conception and design of the study, and/or data acquisition, analysis and interpretation and/or
- Involved in drafting the manuscript or revising it critically for intellectual content
- Final approval for submission was given by every author
No changes in the authorship will be entertained after submission, unless there is a request with a clear explanation to the satisfaction of the Editorial Board
Manuscript Requirements
CMJ publishes the following types of articles:
- Original Research Articles
- Review Articles
- Case Reports (not more than three in an issue)
- Viewpoint
- Editorials
- Letters to the Editor
Submissions should include a cover letter, title page, abstract, main manuscript and other statements. Generally, the cover letter should provide a brief overview of the article content and why your article is worth publishing. Other details for the cover letter are presented in the Research and Publication Ethics section above. If your word count exceeds the recommended amount, please provide an explanation as to why it does.
The title page should have the title of the article, the corresponding author’s full name, institutional address and email as well as the names, institutional addresses and emails of all co-authors. The title page should also have the word count (excluding the title page, abstract, figures/tables, references).
The main manuscript should include a title, abstract and the main text following the headings outlined in the next section. Headings in the manuscript should follow this format: bold caps, bold lower case, plain text, italics.
Other statements: At the end of the manuscript (before the reference section) acknowledgements, competing interests, ethical approval, funding and author contributions to the article should be stated. If these statements do not apply to your specific article type you may write ‘not applicable’ (eg. Ethical approval: not applicable). Please refer to the Research and Publication Ethics section for specific requirements.
All files should be uploaded as a Word document. The editorial team will convert the files to PDF before sending for peer review. Documents submitted as a PDF file will be returned to the corresponding author.
Please follow the required specifications given below, for the type of article you are submitting.These specifications include lengths (word counts), illustrations, table limits and reference counts. The word count excludes the title page, abstract, tables, acknowledgements, contributions and references. Times New Roman font size 12 is the preferred font for all submissions. Manuscripts should be as succinct as possible without unnecessary circumlocution.
Manuscripts should generally have the following structure:
- Introduction should introduce the background subject of the study to the reader in clear language with supporting evidence.
- Methods must include a statement regarding approval from Institutional Review Board.
- Results should be presented in logical order and repetition of the same information in text as well as tables and figures must be avoided.
- Discussion should not just repeat the results, but compare the current findings with the existing literature.
- Conclusions should be derived from the findings of the study and not overarching ones.
Avoid recommendations such as ‘further study is required’ – further studies are always needed and will be invariably conducted in the subject. Every subheading (Abstract, Introduction, Methods, Results, Discussion, References, each table, each figure (with captions), Acknowledgements) must start in a fresh page.
Guidelines specific to article type
Please strictly adhere to the following instructions for the type of submission in order to avoid delays in review and publication:
Original Research Articles
An Original Research Article must be a report of an original research conducted after the necessary approval from the Institutional Review Board and must be presented in the following structure:
- Title page with authors information
- Structured abstract [Objective, Methods, Results, Conclusion]: 250 words
- Introduction, Methods, Results, Discussion (including limitations and conclusion)
- Word count: 4,000 words
- Tables/figures: 5 each
- References: 40
Review Articles
Both narrative and systematic reviews are welcome in any branch of medicine and dentistry. Narrative review should adhere to the following format:
- Thorough assessment of the existing literature
- A search strategy
- Description of how articles have been selected
- Analysis of the existing literature including comments on the strengths and weaknesses of the selected studies
- The overall conclusion that can be derived from the review
Systematic reviews should adhere to the following format:
- It should be ideally be presented according to the PRISMA statement
- Ideally it should have a protocol and should have been registered
- It may be preferable to have a more inclusive review with a larger number of studies, when there are sufficient studies, standard analyses including subgroup analyses may be performed to explore group differences
- Cochrane Collaboration risk of bias tool or other tools such as AMSTAR may be used to score the methodological quality
- Heterogeneity should be explained in the discussion of individual studies
- Appropriate statistical analysis including sensitivity analysis may be presented
Review articles must be presented in the following structure:
- Title page with authors information
- Structured abstract [Objective, methods (search), results, conclusion]: 250 words
- Introduction, Methods, Results, Discussion (including limitations & conclusion)
- Word Count: 5000 words
- Tables: 8 tables / Figures: 6
- References: 150
Case Reports
CMJ will be only interested in accepting case reports that fulfill criteria such as:
- shedding light on a possible new pathogenesis of a disease
- medical errors and lessons learnt
- unreported adverse effects of interventions and drugs
- new diagnostic or treatment procedure
- rare or new disease diagnosed and treated
- unusual presentation and/or association of symptomatology
- debunking myths and misconceptions
This can be a single case report or a case-series and must have the following structure:
- Title page with authors information
- Non-structured abstract: 100 words
- Description of the Case(s)
- Discussion including conclusion
- Word Count: 2000 words
- Figures: 2
- References: 10
Viewpoint
‘Viewpoint’ articles are small essays describing a particular subject matter of current interest to the medical and dental fraternity. The author may put forward his or her own opinion on the subject of discussion; however, it must be substantiated by evidence from the published literature
The structure of the ‘viewpoint’ article will be as follows:
- Title page with authors information
- Introductory paragraph with the background description of the subject
- Discussion including conclusion
- Word Count: 1500 words
- Table or a figure (optional): 1
- References: 10
Editorials
Editorials are usually commissioned by the editorial team. Unsolicited editorials may be accepted based on their relevance and importance. Editorials are primarily opinion essays; however, they should be backed up by available evidence.
The structure is as follows:
- Word count: 1000 words
- References: 5
- Authorship MUST be restricted to one or a maximum of two.
Letters to the editor
Correspondence to the editor may be either a comment on a previous publication or regarding a subject of current interest. If it is a comment on a previous publication, it should be within a time-frame of 3 months after the original publication.
The structure is as follows:
- Word Count: 500 words
- References: 5
- Authors: 3
Short Report
Short reports are short articles focusing on experimental work, innovative methods, audits or preliminary/pilot work. The report must also include an ethical approval statement.
The structure is as follows:
- Abstract: 250 words
- Word count: 2000 words
- Tables/figures: 3
- References: 15
CMJ Reflections
The CMJ Reflections series encourages patients, carers and clinicians to write about their personal experiences of living with illness, caring for those with illnesses as well as their experiences of health services in the Caribbean. These articles are meant to be reflective, engaging pieces to provide insight and awareness on how each person experiences the system in order to help us better understand one another and the system.
Reflections are short passages which are real time experiences. Since they are written for both medical and non-medical readership, they need not strictly include academic language or jargon. These articles will undergo editorial review (and peer-review as necessary). Controversial and ‘propaganda’ types of articles will not be entertained”. The word count for these articles is 600 words. References may be included when appropriate.
References provided in this format are converted during the production process to superscript type. Please note that if references are not cited in order the manuscript may be returned for amendment before it is passed on to the Editor for review.
Quality Improvement (QI) Projects
Quality Improvement projects (QI) involve a systematic approach to improve healthcare problems, delivery of services, patient experiences and outcomes. Authors wishing to submit QI studies should follow the SQUIRE guidelines (https://www.equator-network.org/reporting-guidelines/squire/).
Further support on how to write a QI project can be found here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4857497/ and https://qualitysafety.bmj.com/content/22/9/777
An example of a QI project can be found here: https://bmjopenquality.bmj.com/content/5/1/u210706.w4335.full
A general guide is as follows:
- Concise title
- Unstructured abstract (250 words)
- Word count- 2000 words
- Description of the Problem
-
- summarise the problem being studied
- provide details of the local context and setting
- include a rationale for the study
- existing evidence on the problem, any previous studies conducted
- what interventions worked, what did not work.
- This section should end with the project aim (preferably using SMART principles eg. The aim of the project was to reduce time from triage to ECG for all patients presenting with chest pain from 30 minutes to 10 minutes, over a 6 month period)
- Design
-
- important aspects of the setting relevant to the intervention
- description of the intervention and why it was chosen
- what approach was used to test the intervention
- what measures were used to assess impact including balancing measures
- who comprised the study team
- Plan
-
- describe what was exactly done
- what specific QI method was used eg PDSA cycle
- how was data analysed
- Findings
-
- what was found
- how do you know the intervention led to any results
- Implications
-
- what do your findings mean
- relate to the aim
- strengths and limitations
- lessons learned
- recommendations
- sustainability
- scaling up
Clinical audit
A clinical audit is used to determine if the quality of a current service meets existing pre-determined standards. Service evaluation simply tells you the current state of a service. Submissions reporting clinical audits should be 1000-1500 words and should generally have the following structure:
- Title
- Background: rationale for topic and include relevant background information
- Aim (criteria to be measured): what you want to achieve.
Eg. Diabetic patients should have HbA1c tested at least once a year
- Standards: You must state the standards used to compare your practice against (Use existing evidence based international or local guidelines)
Eg. 80% of diabetic patients should have HbA1C tested at least once year
- Method: Describe what data was collected, how it was collected and how it was analysed.
- Results: Describe what the data tells you about current practice and how it compared to the standards identified.
- Recommendations: Describe any suggestions for improvement.
What is the difference among research, audit and service evaluation?
- Research generates knew knowledge or adds to existing knowledge in an area
- Audit measures a clinical practice against pre-determined standards
- Service evaluation assess the quality of a current clinical service
This article is useful to help understand the differences: https://ebn.bmj.com/content/17/3/65
Ethical considerations for audits and service evaluation
Ethical standards apply to audits and service evaluation as well. Some audits may require interactions with human subjects which may include charts containing private medical information. Hence, researchers should not assume that a study they are conducting is only an audit and does not require formal Ethics Committee approval. Rather, they should seek advice from relevant authorities and/or Ethics Committees to ensure that their projects do not fall under the category of ‘research’.
References
The references must be in chronological order in the text, quoted in Arabic numerals within square brackets before the full-stop.References provided in this format are converted during the production process to superscript type. Please note that if references are not cited in order the manuscript may be returned for amendment before it is passed on to the Editor for review.
The list of references must be in the same order at the end of the main text (after conclusions in the ‘Discussion’ section, started in a new page).
Examples:
- Multi-author article published in print
Author GH, Author IJ. Title of paper. Journal Title in Full in Italics 2018; 12: 111-2.
- Multi-author article published e-pub ahead of print
Author GH, Author IJ. Title of paper published as ‘ePub ahead of print’. Journal Title in Full in Italics 2018 Jan 31; doi: 01.2345/012.345678.
- Chapter in a Book
Author GH, Author IJ, Author KL, et al. Chapter title. In: Editor MN, Editor OP, eds. Title of Book. Place: Publisher, 2018: 123-45.
- Single-author book
Author GH. Book Title, 5th Ed. Place: Publisher, 2018.
- Website
Author(s) of website. Title of document/page, 2018.
www.URL.com/link.pdf (Accessed 31/01/2018).
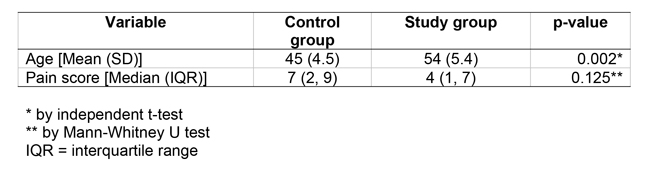
Tables
Tables should be following the “References” section, included in the same file. Each table must have a title above it, numbered consecutively in Arabic numerals. The caption should be as concise as possible and not too long, but providing adequate information regarding the contents of the table. The legends explaining abbreviations, inferential statistical tests etc., should be provided at the bottom of each table.
Please provide the exact p-value; please do not provide “p<0.000” or any such information directly extracted from the pivot table of the statistical software.
Each table must be presented on a separate page.
The table format shall be as follows (example):
Table 1. Comparison of patient variables
Figures
Images and graphs may be submitted as embedded components in the same file as the text, presented after the tables. The figures should have a brief caption and presented consecutively numbered using Arabic numerals.
In graphs, unnecessary use of 3-D images (pie charts/histograms) should be avoided. Photographic images must be of JPEG format, of minimum 300 pixels per inch resolution. The fonts used in the graphs should be the same as the text. The caption should be as concise as possible and not long, but providing adequate information regarding the contents of the table. The legends explaining abbreviations should be provided at the bottom.
Submission process
Manuscripts should be submitted using the online submission portal. All documents should be uploaded as Word documents. Authors will be notified by email that their manuscript has been received.
3. Post Manuscript Submission
Peer review process
Each submission is screened by a deputy editor. At this stage, they may choose to decline the manuscript if it is thought that it does not fit with the journal’s aims and scope or if considered unsuitable in terms of quality.
If the manuscript is considered suitable, it is then moved to the second stage. Each manuscript is reviewed by two independent external peer reviewers. Once the peer reviewers provide their feedback, the assigned deputy editor reviews the feedback and providers his/her additional comments. Recommendations can be to a) decline the manuscript b) conditional acceptance where either major or minor revisions are required and c) accept the manuscript.
Once the decision is made, the decision letter is emailed to the author informing him/her of the decision. If revisions are required, the author is given a specified time period in which to re-submit the revised manuscript. The manuscript will either be reviewed again by the deputy editors or peer review requested again if thought necessary.
The Editor-in-Chief makes the final decision on all manuscripts. All decisions will be communicated with the corresponding author by email. The processing time of a manuscript varies depending on the complexity of the subject area and availability of suitable peer reviewers.
Instructions for Reviewers
CMJ operates on a single blind peer review process. This means that the peer reviewer’s identify is unknown to the authors. Reviewers should declare any competing interests prior to accepting to review a manuscript. Reviewers are sent a peer review template to guide them through the process and are asked to read the instructions to authors information as well.
We urge all peer reviewers to use this template in order to provide authors with sufficient feedback on their paper. Reviewers are also reminded that all manuscripts under review are confidential.
Copyright
Author(s) retain copyright and full publishing rights of their published articles without restrictions and grant to the publisher non-exclusive right to publish their articles, to be cited as its original publisher in case of reuse, and to distribute it in all forms and media.
Copyediting
Manuscripts accepted for publication will be edited by the journal editorial team and sent to the corresponding author for approval of the final version. In order to expedite the process, authors are asked to review the manuscripts and return the approved version by the date requested from the journal editorial team.

