Authors:
Ms. Tashanna Aubin1, Dr. Andrea Joseph1, Mr Naveen Ratan2,3
1The University of the West Indies, St. Augustine Campus, Faculty of Science and Technology, Department of Physics
2Southern Medical Clinic
3The University of the West Indies, Department of Clinical Medical Sciences/Department of Physics (Part-Time)
Corresponding Author:
Ms Tashanna Aubin
The University of the West Indies
St. Augustine Campus
Faculty of Science and Technology
Department of Physics
[email protected]
DOAJ: fc99a5a7f4d44492bbd19ca06621959a
DOI:
Copyright: This is an open-access article under the terms of the Creative Commons Attribution License which permits use, distribution, and reproduction in any medium, provided the original work is properly cited.
©2023 The Authors. Caribbean Medical Journal published by Trinidad & Tobago Medical Association
Abstract
Objectives
In Trinidad and Tobago, there is currently no legislation in place to protect patients, staff and the general public regarding the use of ionizing radiation and radioactive sources. One commonly used radioactive source is the isotope Iodine-131 (131I) which plays a key role in the treatment of thyroid disorders and malignancies. This study aimed to investigate the contamination levels of patients, contact surfaces at their homes and solid waste accumulated post-administration of 131I. The data obtained was used to assess relative risks associated with current practices and to determine if there is a need for the implementation of radiation isolation wards.
Methods
The homes of 19 patients were surveyed post-administration with the use of a calibrated Geiger Muller detector. The dose rate of the patients was measured from a distance of 1 m. Contact surfaces in the isolation area were measured together with the weight and exposure of the solid waste generated by the patients.
Results
26% of patients had dose rates that were higher than the average international release criteria. All outpatients produced a total of 18.54 kg of contaminated solid waste with a total dose rate of 172.608 µSv/hr during the first 48-72 hours post-treatment.
Conclusion
It was determined that the highest relative risk was related primarily to the lack of monitoring of outpatients. It can be concluded that outpatient treatment with 131I is safe in Trinidad and Tobago once the patients’ home is suitable for isolation and clear guidelines on radiation protection measures are discussed.
Introduction
Globally, thyroid cancer has been named the most common type of endocrine cancer, and statistics have shown that over the past three decades, its incidence rate has been increasing continuously. 1, 2 This has been associated with the increased detection of the disease and may not necessarily be an increase in the actual incidence rates. 3,4 Even though a significant increase has been observed, the mortality rates significantly declined in most countries or remained stable at very low levels [5,6]. The risk factors for this type of cancer include gender, age, body mass index, weight, the presence of diabetes, lifestyle, genetic factors, hormonal factors, nutritional factors, exposure to ionising radiation and use of certain drugs. 7-10 The treatment options available to thyroid cancer patients include surgery (a partial or complete thyroidectomy), radioactive iodine (131I) treatment and external beam radiation. 11 There has been great improvement in the ability to optimize the treatment of patients with individualized therapeutic procedures using 131I. 12
Some countries consider the administered dose while others, the retained dose of the patients when determining their patient release criteria. 13 The treatment goals are to remove or reduce and destroy thyroid tissue, often achieved by having a thyroidectomy followed by radioactive ablation therapy using 131I .11 The treatment of 131I can be safely administered on an outpatient basis when patient release criteria are met as it is essential to reduce the risk of radiation exposure to the general public and family members of the outpatient. 14-16
In a study done to determine the exposure to radiation of family members of outpatients who were treated with 131I for hyperthyroidism, it was seen that 97% of adult family members complied with instructions and were below the dose limit. 16 It was also seen that 89% of child family members were within acceptable limits. Based on the data collected, it was concluded that patients treated with 131I for hyperthyroidism were suitable candidates for the treatment to be administered on an outpatient basis. It was also noted that admission to a hospital isolation ward was not required as exposure rates were within acceptable limits. A similar study was conducted for thyroid cancer patients who were treated with 131I. 17 Their results showed that there were no levels of contamination above those specified by the Nuclear Regulatory Commission (NRC). 18 It was concluded that overall patient satisfaction and comfort levels were much higher for persons receiving outpatient treatment in comparison to the patients who were treated, and then confined to radiation isolation wards. The cost associated with treatment for outpatients, when compared to inpatient treatment, was found to be considerably lower. Research was further conducted to determine the exposure to the caregivers of outpatients treated with high doses of 131I . 19 Nantajit and colleagues acknowledged the benefits of utilising the guidelines that were dose limit-based in comparison to the previously utilised activity-based patient release criteria. The results showed that the radiation exposure to the caregivers was below the public dose limit of 1 mSv. 20 This confirmed the theory that the outpatient treatment plan was safe and a widely accepted option. They also recommended such a treatment plan for countries where hospitals have limited resources.
One study conducted in Trinidad and Tobago noted that treatment options with radioiodine were well received and based on international guidelines. 21 Another study documented the pattern of thyroid disease in Trinidad. 22 However, little to no research has been done in Trinidad and Tobago about the safety and practices of outpatient treatment with radiopharmaceuticals. As a result, radiation contamination to family members, caregivers and pets, if any, remains unexplored. The objectives of this research were to determine the levels of radiation-induced contamination within the homes of outpatients treated with 131I, to determine the amount of radioactive trash generated by the patient post-treatment and to assess the safety of the outpatient procedures by performing a failure mode effect analysis.
Methods
This research was conducted as a prospective observational study utilising a consecutive case series and follows similar work by Panzegrau and colleagues, Nantajit and colleagues and Ibis and colleagues. 17,19,23 It was designed to include all willing participants of thyroid outpatient cases, based on convenience sampling, who were treated with 131I from March 2019 to October 2020. This resulted in a sample size of 19 patients, comparable to the international studies cited.
Ethics approval was received from the Campus Research Ethics Committee of the University of the West Indies and approval for the research was granted by two private health institutions. The patients were recruited using approved patient lists and contacted via telephone to discuss the requirements for inclusion in the study. For some patients, the informed consent process took place before they were administered their treatment doses of 131I. Due to the COVID-19 restrictions, the informed consent process was done over the telephone for some patients. It was also reiterated to the patients that they were able to withdraw from the study at any time and without penalty. The inclusion criteria are given below:
- Patients must have received outpatient 131I therapy for hyperthyroidism/Greaves disease or thyroid cancer.
- Patients must be 18 years or older at the time of treatment or have parental consent.
- The patient must have been treated within 72 hours before the radiation-contamination survey.
The collection of data for this research project occurred 48-72 hours post-treatment of the patient depending on their administered dose. For patients who were treated with an activity of 75 mCi or less, home visits were scheduled for 48 hours after the administration of 131I. For patients treated with an activity of 75 mCi and above, home visits were scheduled for 72 hours after 131I treatment. Firstly, a background radiation reading was obtained, and a sketch of the patient’s home was made of the areas of interest before taking measurements of the potentially contaminated areas. During the contamination survey, the detector was held close to the different surfaces to obtain the measurements. All exposure rates were measured using a RADEX Geiger-Muller radiation detector. Readings were recorded in terms of dose rate (µSv/hr) on a coded survey sheet considering the background radiation. The exposure rate using the patient as a point source was also measured at a distance of 1 m.
The patients’ garbage was weighed using a handheld digital scale and the exposure rate of the bag was determined. The trash included but was not limited to, all the solid waste generated by the patient during the isolation period.
During the home visits, the researcher was outfitted with the required disposable PPE to reduce the possibility of exposure to any contamination and to prevent any spread of surface contamination during the survey. The PPE was similar to that used by nurses and medical staff when attending to patients in isolation rooms and included a water-repellent isolation coverall, surgical gloves, waterproof shoe covers with closed-toe shoes and a disposable surgical mask. A personal radiation direct-reading dosimeter, Radex ONE, with the capability to detect β (Beta), γ (Gamma) and X radiation, was also worn.
The instrument used in the data collection process of the radiation survey was a portable Radex RD1706 Geiger-Muller counter with a detection range of 0.05 to 999.0 µSv/h. The device was calibrated to international standards and verified by the calibration certificate issued by QuartaRad. Before each use for a contamination survey, as noted earlier, a background reading was taken which was subsequently subtracted from each exposure reading recorded. For precision, the device takes a reading every 10 seconds to give a standardised reading in 40 seconds.
To determine the total effective dose equivalent (TEDE) of household family members and members of the public, the guidelines in the IAEA Safety Reports and NCRP Report No. 37 were used to calculate the range of equivalent doses corresponding to all the treatment activities of the patients who participated in this study. 24-29 The regulatory guides present the following equation (Equation 1) as one method for calculating the estimated TEDE of the most exposed person. Such a person is allowed to receive 5 mSv (500 mrem) on the theory that that person derives some benefit from the exposure, and is often a spouse, parent, adult child or friend, or caregiver.
D(∞)= 34.6 Γ Qο Tρ E
r2
where
D(∞) – dose from external exposure to gamma radiation (TEDE)
34.6 – conversion factor of 24 hrs/day times the total integration of decay (1.44)
Γ – specific gamma-ray constant for a point source, R/mCi.hr at 1 cm
Qο – the initial activity of the point source in millicuries, at the time of the release
Tρ – physical half‐life in days
r – distance from the point source to the point of interest in centimetres
Ε – occupancy factor
For 131I, is 2.2 R·cm2/mCi·hr, Tρ is 8.04 days. Ε is given a value of 0.25 since the half-life is longer than 24 hours and the patient can adhere to the special instructions that are given to maintain this occupancy factor.
Results
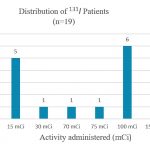
Nineteen radiation contamination surveys were completed with eighteen unique subjects. For patient confidentiality, the data collected in this research was deidentified and coded. The distribution of patient treatment activities is given in Figure 1. To analyse the data collected, three treatment activity ranges were utilised: Group 1 (8 subjects) comprising treatment activities of 3-30 mCi; Group 2 (8 subjects), activities of 75-100 mCi; and Group 3 (3 subjects), activities of 150 mCi.
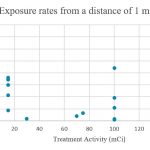
Presented in Figure 2 are the patients’ exposure rates at 1 m. While it was expected that as the patient dose increased, the exposure rate would also have increased, it was noted that high patient exposure rates were recorded for patients with low doses of 131I. Similarly, there were patients receiving high doses of 131I with low readings of the exposure rate at 1 m.
Table 1 shows the measurements of the weight and activity of each patient’s solid garbage. While most patients had garbage activity levels below 5 µSv/hr, there were three abnormally high readings of exposure. These patients were all advised not to put their garbage out for collection by the regional corporations but were instructed to secure it within their homes for an additional 1-2 weeks. The highest reading of 68.8 µSv/hr was measured for 3.5 kg of solid waste. This patient’s solid waste was also the highest volume of garbage produced within the 2-3 days post-treatment. No reading was taken for patient 14 as this patient’s garbage was accidentally discarded before the radiation survey.
Table 1: Weight and Activity of Patients’ radioactive waste
| Patient ID # | Garbage Weight
(kg) |
Garbage Activity
(µSv/hr) |
| 1 | 1.130 | 0.160 |
| 2 | 0.370 | 0.110 |
| 3 | 0.910 | 0.490 |
| 4 | 0.930 | 1.330 |
| 5 | 0.200 | 0.300 |
| 6 | 0.930 | 0.560 |
| 7 | 0.300 | 0.388 |
| 8 | 0.680 | 5.600 |
| 9 | 3.000 | 1.530 |
| 10 | 0.750 | 0.330 |
| 11 | 0.400 | 3.020 |
| 12 | 0.380 | 0.260 |
| 13 | 3.500 | 58.800 |
| 14 | – | – |
| 15 | 1.200 | 37.900 |
| 16 | 0.760 | 45.210 |
| 17 | 1.000 | 2.270 |
| 18 | 0.200 | 0.740 |
| 19 | 1.900 | 3.610 |
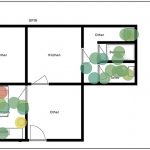
Radiation heat maps were generated for all patients using the Tableau data visualization software, three of which are presented in Figures 3-5. The radiation heat maps show the distribution of the exposure rates measured in the patients’ isolation area and frequently touched surfaces. The variation in colours is used to indicate the radiation levels in the contaminated areas, where shades of green represent lower exposure rates and shades of red represent higher exposure rates. The map in Figure 3 corresponds to a patient treated with 15 mCi of 131I for hyperthyroidism. From the map, the area of greatest contamination was on the patient’s pillow due to a combination of saliva and sweat. Figure 4 illustrates the radiation contamination heat map for a patient administered with 150 mCi of 131I and shows high levels of radioactivity in both the patient’s laundry basket and garbage bin. This patient was instructed to isolate for three weeks due to the high administered activity and opted to use disposable plates and cutlery. The map of Figure 5 corresponds to a patient receiving a therapeutic dose of 30 mCi 131I and gives a good indication that the patient spent much time in the chair adjacent to the bed during the isolation period. The levels of radioactivity in the shower area are normal and there is increased radioactivity in the predicted areas, the toilet and the sink of the face basin.
Using equation (1), the calculations of total effective dose equivalent (TEDE) were tabulated and can be found in Table 2. The patients’ treatment activities were used in the calculations and the results show that for activities above 30 mCi, the effective dose is greater than 5 mSv. Also, the calculations show that patients receiving 3 mCi do not require both verbal and written instructions as the effective dose to the most exposed person is less than 1 mSv.
Table 2: Calculations of TEDE of 131I Outpatients
| Treatment Activity (mCi) | Treatment Activity (MBq) | Total Effective Dose (rem) | Total Effective Dose (mSv) |
| 3 | 111 | 0.05 | 0.5 |
| 15 | 555 | 0.23 | 2.3 |
| 30 | 1110 | 0.46 | 4.6 |
| 75 | 2775 | 1.15 | 11.5 |
| 100 | 3700 | 1.52 | 15.2 |
| 150 | 5500 | 2.28 | 22.8 |
Table 3 shows the Failure Mode Effect Analysis for the process involved with administering a patient the radiopharmaceutical 131I. This table shows the relative risk associated with the steps of the procedure and the probable failure modes by the calculation of a Risk Priority Number (RPN) [30]. The RPN is a product of the severity (S), frequency of occurrence (O), and probability of detection (D). This table identifies the activities of outpatient treatment with the highest risk.
Table 3: Failure Mode Effect Analysis
|
PROCESS |
POTENTIAL FAILURE MODE |
POTENTIAL CAUSE OF FAILURE |
POTENTIAL EFFECT OF FAILURE |
O |
S |
D |
RPN= |
|
Administration of
|
The patient does not arrive or arrives late to their appointment/ administration time |
The patient must be rescheduled |
The patient may be under-dosed due to radioactive decay. Treatment may be less effective than desired. |
2 |
4 |
1 |
8 |
|
The patient is given the wrong dose |
Lack of measuring equipment or qualified personnel | The patient may be over-dosed | 2 | 8 | 4 | 64 | |
| The patient may be under-dosed | 4 | 5 | 4 | 80 | |||
|
Insufficient radiation protection protocols and release criteria |
The wrong patient is given the wrong dose (Misadministration) |
3 |
5 |
8 |
120 |
||
| Staff members/ family members/ members of the public reaching or exceeding annual dose limits |
5 |
6 |
5 |
150 |
|||
|
The patient is released after treatment to isolate at home |
The patient does not adhere to all protocols and interacts with family members as usual |
The patient was not adequately informed of the radiation risk to household members |
Family members reaching or exceeding the annual dose limit |
3 |
8 |
8 |
192 |
|
Living arrangements unsuitable for isolation |
Family members reaching or exceeding the annual dose limit | 4 | 8 | 8 | 256 | ||
| Pregnant women or children 12 years and under may be exposed to radiation (external/internal) |
4 |
9 |
9 |
324 |
|||
|
The patient does not adhere to any protocols and leaves home during isolation |
The patient was not given proper isolation guidelines and timelines to resume regular activities | Household members/ members of the public may reach or exceed annual dose limits if the patient stops restrictions too soon |
4 |
8 |
9 |
288 |
|
| The patient goes back out to work before isolation ends | Depending on the patient’s job, special groups like pregnant women and children may be exposed |
5 |
7 |
8 |
280 |
Figure 4: Radiation Heat map showing patient treated with 150 mCi for thyroid cancer

Figure 5: Radiation heat map showing patient treated with 15 mCi for hyperthyroidism with a high exposure rate

Discussion
Out of a total of 19 radiation contamination surveys conducted, eleven subjects (58%) received ablative doses of 75–150 mCi and eight subjects (42%) received a diagnostic or therapeutic dose of 3–30 mCi. The distribution of patient treatment activities in Fig 1 made it possible to group patients for comparative analysis. In comparing the trends seen in Groups 2 and 3 (with activities ranging from 75 mCi to 150 mCi), from the exposure rates at 1 m in Fig 2, it is observed that some of the patients who were treated with low doses of 131I had higher exposure rate readings than patients who were treated with higher doses of 131I. This pattern does not follow any direct or inverse proportional relationships as anticipated. 27 For instance, one patient who was treated with 15 mCi had an exposure rate of 36 µSv/hr at 1 m, 48 hrs post-treatment, whereas another patient who was treated with 150 mCi had an exposure rate of 6.85 µSv/hr at 1 m, 72 hrs post-treatment. The presence of an enlarged or overactive thyroid gland in Group 2 (low-dose hyperthyroidism patients) may be the cause of the high exposure rates as there is significantly more uptake of the 131I as compared with Group 3 (high-dose thyroid cancer patients). Patients in Group 3 may have previously had a complete or partial thyroidectomy and so would not have much thyroid tissue left. This may be the cause of some patients treated with high doses of 131I having low exposure rate readings at 1 m. Uptake for these patients may have been reduced due to less thyroid tissue present. It is also possible that these patients had faster clearance rates of the administered activity. 27
Contaminated solid waste from patients’ households post-treatment was expected. 28 The level of this contamination, however, varied among patients. Their interpretation and adherence to the radiation safety guidelines and restrictions played a major role in how well they were able to reduce or minimize their contaminated waste. High exposure readings were expected from the waste generated by the outpatients as most of the activity is excreted via bodily fluids in the first three days post-therapy. The patient’s saliva is also radioactive during this period due to uptake in the salivary glands from oral administration, so any inedible food waste, such as chicken bones, fruit seeds and peels, would be contaminated. In this study, a total of 18.54 kg of contaminated solid waste with a total estimated dose rate of 172.608 µSv/hr was produced by outpatients during the first 48-72 hours post-treatment. The patient with the highest contaminated waste of 3.5 kg and a dose rate of 58.8 µSv/hr, was advised to wait for an additional two to three weeks before putting the waste out for collection. One problem that arises with 131I contamination is that because of its half-life, there is no significant decay of contaminated surfaces and objects by the next day. Some of the recommendations to minimize the quantity and radioactivity of the waste are that patients should not use disposable plates and cutlery but instead use regular dishes and wash and reuse the same during their isolation period; they should refrain from consuming food that has inedible parts, for at least a week after treatment due to the high levels of 131I activity remaining in their saliva; and patients should refrain from discarding items of clothing that may have been soiled with bodily fluids but instead secure the waste and delay its disposal in public trash. 29
The use of radiation heat maps was not found in any other research work examined but proved to be a useful tool in visualizing surface contamination. Based on the trends seen in the heat maps, it is advised that the decision to hospitalise or release a patient treated with 131I should be done on an individual basis. The heat map in Fig 3 shows in the floor plan of the isolation area that the patient did not have the sole use of a toilet/bathroom area for the isolation period. This may have resulted in family members being exposed to contamination on contact surfaces. However, as this patient’s treatment activity was 15 mCi, using Equation 1, the most exposed family member would have received an estimated total effective dose equivalent of 2.3 mSv, as seen in Table 2. Based on international guidelines, this outpatient treatment is deemed safe. 26, 27
The heat map in Fig 4 shows the floor plan of a patient treated with 150 mCi. This patient was completely isolated from family members during the isolation period of three weeks. In this case, the calculated TEDE of the most exposed person was 22.8 mSv, as seen in Table 2. However, since the patient accurately followed outpatient protocol, no person was exposed to or received this dose. This patient also had a significantly high reading of contaminated waste due to the frequent use of disposable cutlery, plates, and cups. Due to the high value of the dose rate of the waste and the colour coding of the data visualisation software, the extent of contamination of the toilet and bathroom area cannot be fully appreciated from the heat map in comparison to the other heat maps. It must be noted, though, that while the dose rate was much smaller relative to the waste, contamination was detected in the toilet and bathroom area.
The heat map in Fig 5 shows the floor plan of the isolation area of a patient treated for hyperthyroidism with 15 mCi. Similar to Fig 3, the most exposed family member would have received a TEDE of 2.3 mSv, as seen in Table 2. Based on international guidelines, this outpatient treatment is also deemed safe. The heat map clearly shows that the patient spent much time in the armchair in the isolation area. The contamination may have been due to sweat and saliva as the patient confirmed that meals were also consumed in this chair. It is expected that this contamination would be eliminated over time by physical decay.
In the analysis of international best practices for patient release after a radionuclide treatment, some countries regard dose-rate-based release criteria as the safer option for which an average dose rate of < 26.3 µSv/h at a distance of 1 m is required. 26 Other countries regard activity-based release criteria as the safer option with an average activity of < 710 MBq (19.2 mCi) for patient release. In Table 2, the summary of the calculations using Equation 1 is given. From this, we see that for up to a dose of 30 mCi, the most exposed person would receive an effective dose of 4.6 mSv, which is within the limits of the guidelines. This would include all patients from Group 1 – their outpatient treatment is safe and there is no need for hospitalization in an isolation ward. It is also mentioned that for any administered dose that may result in a total effective dose of any exposed person of ≤ 1 mSv, the released patient must be provided with both verbal and written instructions in keeping with the as low as reasonably achievable (ALARA) principle. Hence some patients in Group 1 would only require either verbal or written instructions as the TEDE was less than 1 mSv.
To quantify the safety of outpatient treatment with 131I, a Failure Mode Effect Analysis (FMEA) was done in Table 3 based on the current practices and associated risk factors. FMEA is typically used to identify vulnerabilities in risk mitigation and enables the identification of the activities with the highest risk and which areas need to be layered with multiple mitigation factors to prevent worst-case scenarios from occurring. While this has not been seen in similar research papers examined, the application of the FMEA in this context allowed the identification of high-priority areas requiring further safety improvements and optimisation in outpatient treatment. The Risk Priority Number (RPN) of all failure modes ranged from 8 to 324, with an average value of 176. As seen in the table, the case in which the patient is released and does not adhere to the isolation guidelines when there are children or pregnant women present in the household had the highest RPN of 324. Most failure modes with high RPN were identified to occur when the patient is released as an outpatient required to isolate at home. This is because their activities and movements are generally not monitored and will most likely go undetected. The steps in the process of 131I treatment administration can allow for the creation of a checklist that can be utilised for the improvement of the entire treatment process. It is recommended that the following should be added: any process step with an average RPN score of 50 or greater, any item with a detection value greater than six and any severity score greater than four. As a result, this checklist could be used for the standardisation of patient release, and the safety of outpatient treatment can be ensured. This FMEA-based checklist can be used to identify the vulnerabilities and mitigation tasks to increase safety in processes and guarantee higher quality control.
In conclusion, in this study, the relative risk of outpatient treatment with 131I was assessed by measuring the contamination levels post-treatment in the outpatients’ isolation areas and calculating the TEDE of the most exposed household members. From the data collected, patient adherence to stipulated guidelines and isolation instructions was high and the TEDE to family members were within the limits of international guidelines. From the Failure Mode Effect Analysis, it was determined that the highest relative risk associated with the current practices of the treatment seemed to be primarily related to the lack of monitoring of outpatients and increased severity due to potential effects of failure going undetected.
It can be concluded that outpatient treatment with 131I is safe in Trinidad and Tobago once the patients’ home is suitable for isolation and clear and precise guidelines on radiation protection measures are discussed with them. With the implementation of new protocols and with some adjustments to current patient guidelines and requirements, this treatment process can be easily standardised across all local institutions administering radiopharmaceuticals. From the data, not all patients would require in-hospital isolation. However, the possibility exists that for some patients with unsuitable living arrangements, a brief stay in an isolation ward may be deemed necessary. Suggested improvements to the treatment process for the optimisation of this outpatient treatment include the use of an eligibility checklist and a home visit to determine suitability before treatment. It is expected that this analysis of radiation-induced contamination of outpatients post 131I treatment for thyroid disease will contribute to the local body of scientific work conducted in nuclear medicine and endocrinology.
Ethics Approval Statement: Ethics approval was granted from the University of the West Indies Campus Research Ethics Committee, St Augustine.
Conflict of Interest Statement: None
Informed Consent statement: Informed consent was obtained from each participant.
Funding statement: This research was self-funded by the corresponding author.
Authors’ Contribution: Tashanna Aubin collected the data, participated in the study design, led the analysis and interpretation of data, and prepared the manuscript. Naveen Ratan conceptualised and supervised the research, and participated in the study design, data analysis and interpretation, and final revision of the manuscript. Andrea Joseph, as the primary investigator, supervised the overall research effort, including the research question selection, study design and attaining relevant approvals, and detailed and final revision of the manuscript.
References
- Pellegriti G, Francesco F, Concetto R, et al. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. Journal of Cancer Epidemiology. 2013.
- Deng Y, Li H, Wang M, et al. Global Burden of Thyroid Cancer From 1990 to 2017. JAMA Netw Open. 2020;3(6):
- Kilfoy BA, Zheng T, Holford TR, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20(5):525-531.
- Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P. Cancer Incidence in Five Continents. IX. Lyon: IARC; 2007.
- Mengmeng L, Juan P. B, and Salvatore V. Long-Term Declines of Thyroid Cancer Mortality: An International Age–Period–Cohort Analysis. 2020.838-846.
- Solis-Pazmino, P; Salazar-Vega, J; Lincango-Naranjo, E; et al. Thyroid cancer overdiagnosis and overtreatment: a cross-sectional study at a thyroid cancer referral center in Ecuador. 2021
- Khazaei, Z, Malihe S, Kamyar M, Hasan N, and Elham Gi. Incidence and Mortality of Cervix Cancer and Their Relationship with the Human Development Index in 185 Countries in the World: An Ecology Study in 2018. Advances in Human Biology 9 (3): 222. 2019
- Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674-1685.
- Khan N, Afaq F, Mukhtar H. Lifestyle as a risk factor for cancer: Evidence from human studies. Cancer Lett. 2010;293(2):133-143. doi:10.1016/j.canlet. 2009.12.013
- Katzke, V; Kaaks, R; Kühn, T. Lifestyle and Cancer Risk. The Cancer Journal: March/April 2015 – Volume 21 – Issue 2 – p 104-110
- Schlumberger M, Catargi B, Borget I, et al. Strategies of Radioiodine Ablation in Patients with Low-Risk Thyroid Cancer. The New England Journal of Medicine. 2012; 366:1663-73
- Nabhan, F, Dedhia, PH, Ringel, MD. Thyroid cancer, recent advances in diagnosis and therapy. J. Cancer. 2021; 149(5): 984– 992.
- Ross, Douglas S. Radioiodine Therapy for Hyperthyroidism. 2011.
- Tuttle, W. K., and P. H. Brown. Applying Nuclear Regulatory Commission Guidelines to the Release of Patients Treated with Sodium Iodine-131. Journal of Nuclear Medicine Technology. 2000. 28 (4): 275–79.
- Okamoto T, Omi Y, Yoshida Y, Horiuchi K, Abe K. Radioactive iodine treatment of papillary thyroid carcinoma in Japan. Gland Surg. 2020;9(5):1698-1707.
- Barrington, S., Michael J., Andrew G, William H., Peter J, David N., Robert J, Stanley B, Paul S. Radiation Exposure of the Families of Outpatients Treated with Radioiodine (Iodine-131) for Hyperthyroidism. European Journal of Nuclear Medicine 26 (7): 686–92.
- Panzegrau, B, L Gordon, and G.H Goudy. Outpatient Therapeutic 131 I for Thyroid Cancer. J Nucl Med Technol 33: 28–30.
- Nuclear Regulatory Commission. NRC: 10 CFR 35.75 Release of Individuals Containing Unsealed Byproduct Material or Implants Containing By-product Material. 2017.
- Nantajit, D, S Saengsuda, P NaNakorn, and Y Saengsuda.High-Dose Radioiodine Outpatient Treatment: An Initial Experience in Thailand. Asia Oceania Journal of Nuclear Medicine & Biology. 2014. 3 (1): 66–71.
- Abdulhasan Kadhim A, Sheikhzadeh P, Farzanefar S, Yavari S, Mousa Jber M, Ay MR. Measurement of Radiation Exposure to Caregivers of Patients with Thyroid Diseases Treated with Iodine-131: A Review. Frontiers Biomed Technol. 2020. 7(3):192-200.
- Teelucksingh S, Akong J, Klijn C, Ramdass M, Naraynsingh V. Management of hyperthyroidism in Trinidad and Tobago. International Journal of Clinical Practice. 2002 Dec;56(10):746-749.14.
- Narayansingh, V, Raju, G C. Thyroid disease in Trinidad. Journal of the Royal College of Surgeons of Edinburgh.1985.
- Ibis, Erkan, Charles R Wilson, B David Collier, Gur Akansel, Ali T Isitman, and Robert G Yoss. 1992. “Iodine- 13 1 Contamination from Thyroid Cancer Patients,” 6.
- Safety Reports Series No. 63. Release of Patients after Radionuclide Therapy. 2009.
- Gerald J Hine. NCRP Report 37, Precautions in the Management of Patients Who Have Received Therapeutic Amounts of Radionuclides. Journal of Nuclear Medicine. 1972
- Release of Patients after Radionuclide Therapy. 2009. Vienna.
- S Nuclear Regulatory Commission. Regulatory Guide 8.39. Release of Patients Administered Radioactive Materials. 1997
- Willegaignon, José, Marcelo Sapienza, Carla Ono, Tomoco Watanabe, Maria Inês Guimarães, Ricardo Gutterres, Maria Helena Marechal, and Carlos Buchpiguel. 2011. “Outpatient Radioiodine Therapy for Thyroid Cancer.” Clinical Nuclear Medicine 36 (6): 440–45.
- Rassiah P, Su FF, Huang YJ, et al. Using failure mode and effects analysis (FMEA) to generate an initial plan check checklist for improved safety in radiation treatment. J Appl Clin Med Phys. 2020;21(8):83-91. doi:10.1002/acm2.12918
- Woodings S. Radiation protection recommendations for I-131 thyrotoxicosis, thyroid cancer and phaeochromocytoma patients. Australas Phys Eng Sci Med. 2004 Sep;27(3):118-28.